Titanium and it’s Uses
Titanium is as strong as steel but much less dense. It is therefore important as an alloying agent with many metals including aluminum, molybdenum and iron. These alloys are mainly used in aircraft, spacecraft and missiles because of their low density and ability to withstand extremes of temperature. They are also used in golf clubs, laptops, bicycles and crutches.
Power plant condensers use titanium pipes because of their resistance to corrosion. Because titanium has excellent resistance to corrosion in seawater, it is used in desalination plants and to protect the hulls of ships, submarines and other structures exposed to seawater.
Titanium metal connects well with bone, so it has found surgical applications such as in joint replacements (especially hip joints) and tooth implants.
The largest use of titanium is in the form of titanium(IV) oxide. It is extensively used as a pigment in house paint, artists’ paint, plastics, enamels and paper. It is a bright white pigment with excellent covering power. It is also a good reflector of infrared radiation and so is used in solar observatories where heat causes poor visibility.
Titanium(IV) oxide is used in sunscreens because it prevents UV light from reaching the skin. Nanoparticles of titanium(IV) oxide appear invisible when applied to the skin.

History of Titanium
Titanium was discovered by British pastor William Gregor in 1791. The oxide was independently rediscovered in 1795 by Prussian chemist Martin Heinrich Klaproth in rutile from Boinik (the German name of Bajmócska), a village in Hungary (now Bojničky in Slovakia). Klaproth found that it contained a new element and named it for the Titans of Greek mythology.
Pure metallic titanium (99.9%) was first prepared in 1910 by Matthew A. Hunter by heating TiCl with sodium at 700–800 °C under great pressure in a batch process known as the Hunter process. Titanium metal was not used outside the laboratory until 1932 when William Justin Kroll produced it by reducing titanium tetrachloride (TiCl) with calcium. Eight years later he refined this process with magnesium and with sodium in what became known as the Kroll process. Although research continues to seek cheaper and more efficient processes, the Kroll process is still used for commercial production.
What is Titanium?
Titanium is a chemical element with the symbol Ti and atomic number 22. Its atomic weight is 47.867 measured in daltons. It is a lustrous transition metal with a silver colour, low density, and high strength, resistant to corrosion.
It is hard to find titanium as a pure element. However; titanium is found in parts of minerals as compounds in Earth’s crust. Titanium ranks the seventh most abundant metal and the ninth most abundant element in the Earth’s crust. Rutile and ilmenite are the minerals that are considered the best source of titanium. Canada, Australia, and South Africa are the top producing countries. Titanium dioxide is the most common compound of titanium.

Titanium Metal Applications
Aerospace and Marine

- Because titanium alloys have high tensile strength to density ratio, high corrosion resistance, fatigue resistance, high crack resistance,and the ability to withstand moderately high temperatures without creeping, they are used in aircraft, armour plating, naval ships, spacecraft, and missiles.
- For these applications, titanium is alloyed with aluminum, zirconium, nickel, vanadium, and other elements to manufacture a variety of components including critical structural parts, firewalls, landing gear, exhaust ducts (helicopters), and hydraulic systems. In fact, about two-thirds of all titanium metal produced is used in aircraft engines and frames. The titanium 6AL-4V alloy accounts for almost 50% of all alloys used in aircraft applications.
Industrial

- Welded titanium pipe and process equipment (heat exchangers, tanks, process vessels, valves) are used in the chemical and petrochemical industries primarily for corrosion resistance. Specific alloys are used in oil and gas downhole applications and nickel hydrometallurgy for their high strength, corrosion resistance, or both. The pulp and paper industry uses titanium in process equipment exposed to corrosive media, such as sodium hypochlorite wet chlorine gas. Other applications include ultrasonic welding, wave soldering, sputtering targets.
- Titanium tetrachloride, a colourless liquid, is important as an intermediate in the process of making TiO2 and is also used to produce the Ziegler–Natta catalyst. Titanium tetrachloride is also used to iridize glass and, because it fumes strongly in moist air, it is used to make smoke screens.
Consumer

- Titanium metal is used in automotive applications, particularly in automobile and motorcycle racing where low weight and high strength and rigidity are critical.
- Titanium is used in many sporting goods: tennis rackets, golf clubs, lacrosse stick shafts; cricket, hockey, lacrosse, football helmet grills, and bicycle frames and components. Although not a mainstream material for bicycle production, titanium bikes have been used by racing teams and adventure cyclists.
- Titanium alloys are used in spectacle frames that are rather expensive but highly durable, long-lasting, lightweight, and cause no skin allergies. Many backpackers use titanium equipment, including cookware, eating utensils, lanterns, and tent stakes. Though slightly more expensive than traditional steel or aluminum alternatives, titanium products can be significantly lighter without compromising strength. Titanium horseshoes are preferred to steel by farriers because they are lighter and more durable.
Medical
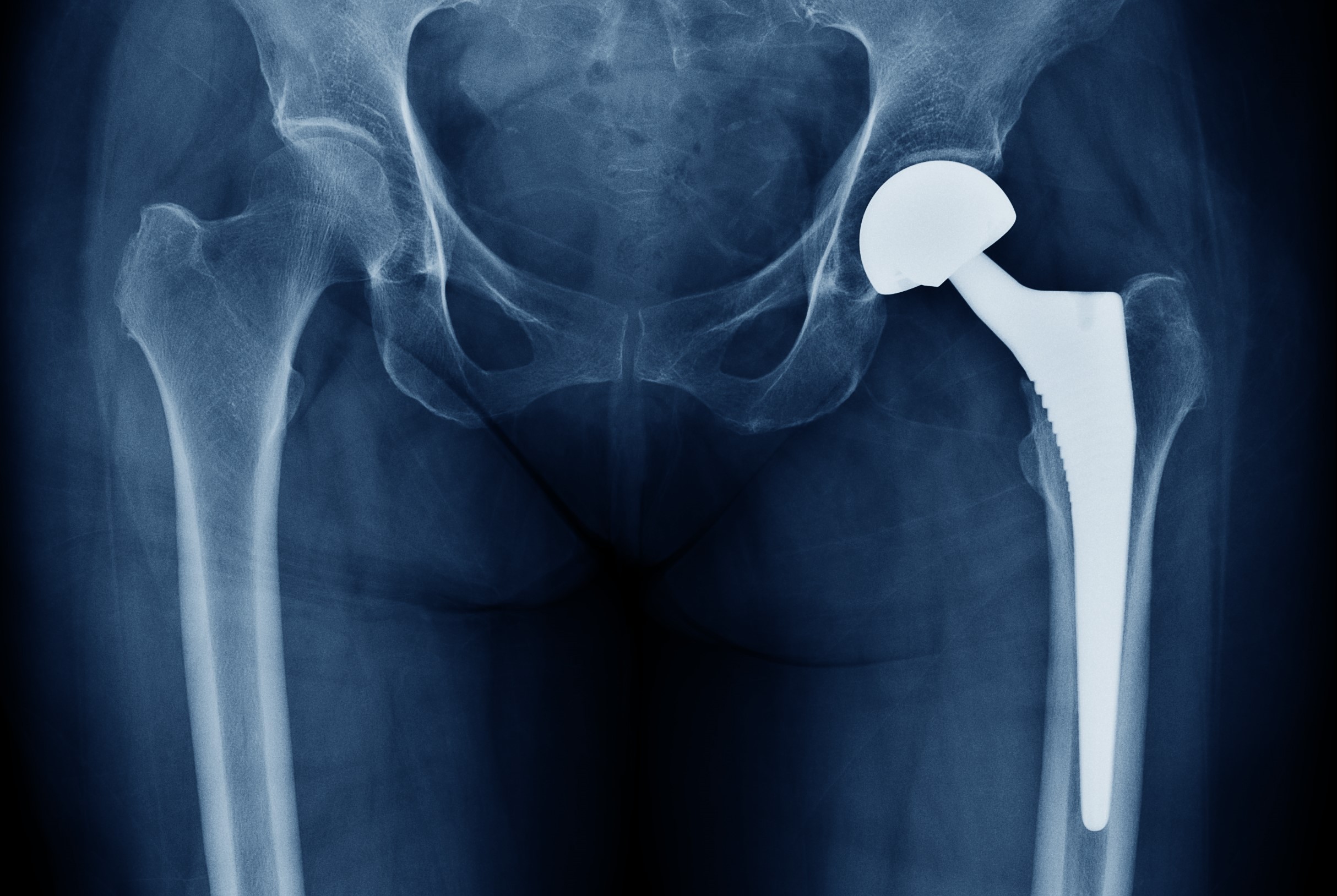
- Because titanium is biocompatible, non-toxic and not rejected by the body, it has many medical uses, including surgical implements and implants, such as hip balls and sockets (joint replacement) and dental implants that can stay in place for up to 20 years. The titanium is often alloyed with about 4% aluminum or 6% Al and 4% vanadium.
- Titanium has the inherent ability to osseointegrate, enabling use in dental implants that can last for over 30 years. This property is also useful for orthopedic implant applications. These benefit from titanium’s lower modulus of elasticity to more closely match that of the bone that such devices are intended to repair.
- Titanium is used for the surgical instruments used in image-guided surgery, as well as wheelchairs, crutches, and any other products where high strength and low weight are desirable.
- Titanium dioxide nanoparticles are widely used in electronics and the delivery of pharmaceuticals and cosmetics.
