Vanadium and it’s Uses
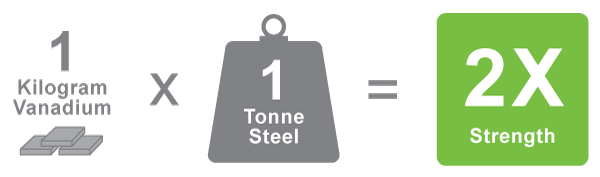
Vanadium is a critical metal predominantly utilized in steel applications. As global mandates shift to more sustainable practices and materials, awareness of the green benefits of vanadium continue to grow.. Vanadium mitigates the cost and carbon footprint across numerous value and supply chains. As a highly versatile metal alloy, vanadium imparts up to twice the strength in many steel applications. Vanadium is also considered invaluable in aerospace and many emerging applications. A renewable energy storage technology known as the vanadium redox flow battery “VRFB”, was invented in 1985 by Maria Skyllas-Kazacos and her team at the University of New South Wales, and represents a compelling green application for vanadium in the future.

History of Vanadium
Vanadium was officially discovered by the Swedish scientist Sefstrom in 1831. He named it after Vanadis the “Swedish Goddess of Beauty and Fertility” because of the attractive brilliant colors of the chemical compounds in which it was first found. It was well named for it has provided many discoveries for scientists and technologists who, for over 150 years, have developed and continue to develop new materials for the benefit of humanity.
The use of vanadium goes as far back as the 3rd Century BC when super strength “Damascus steel” was first manufactured. The first wide-scale use of vanadium in the industry was in 1905 when Henry Ford realized that the Model-T could be stronger and lighter if he used vanadium-enriched steel. The need for stronger, lighter-weight steel emerged with the need for higher safety.
What is Vanadium?
Vanadium is a grey, soft, and ductile high-value metal with several unique characteristics that position it strongly in the steel, alloys, and chemical sectors. The metal also acts as a battery material that is 100% reusable.
More than 85% of vanadium is recovered from magnetite and titano-magnetite ores, either as the primary product or more commonly as a co-product with iron processed for steel production. It can also be recovered as a secondary product from fly ash, petroleum residues, alumina slag, and from the recycling of spent catalysts used in some crude oil refining.
Conventional Applications
Conventional application summary
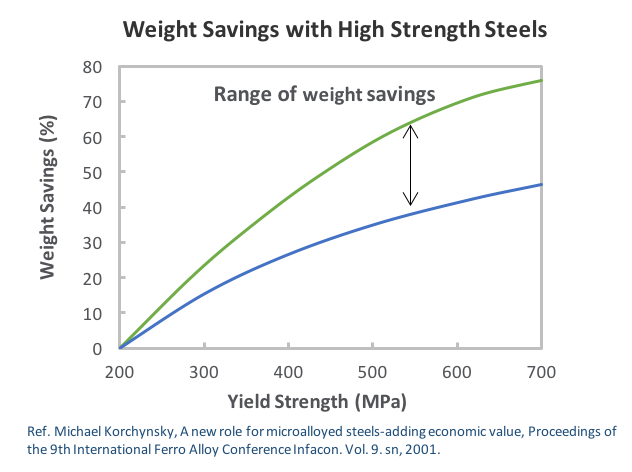
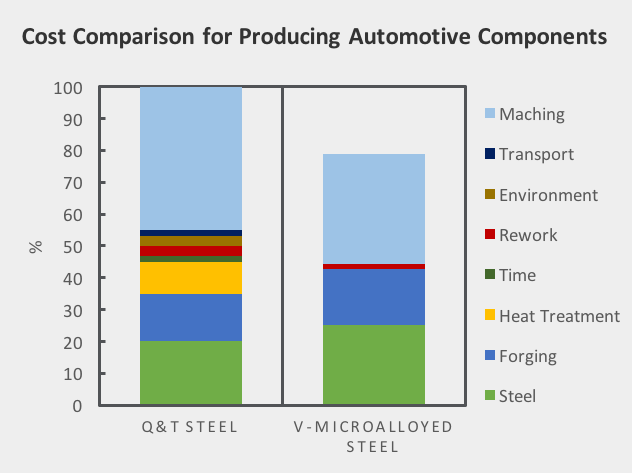
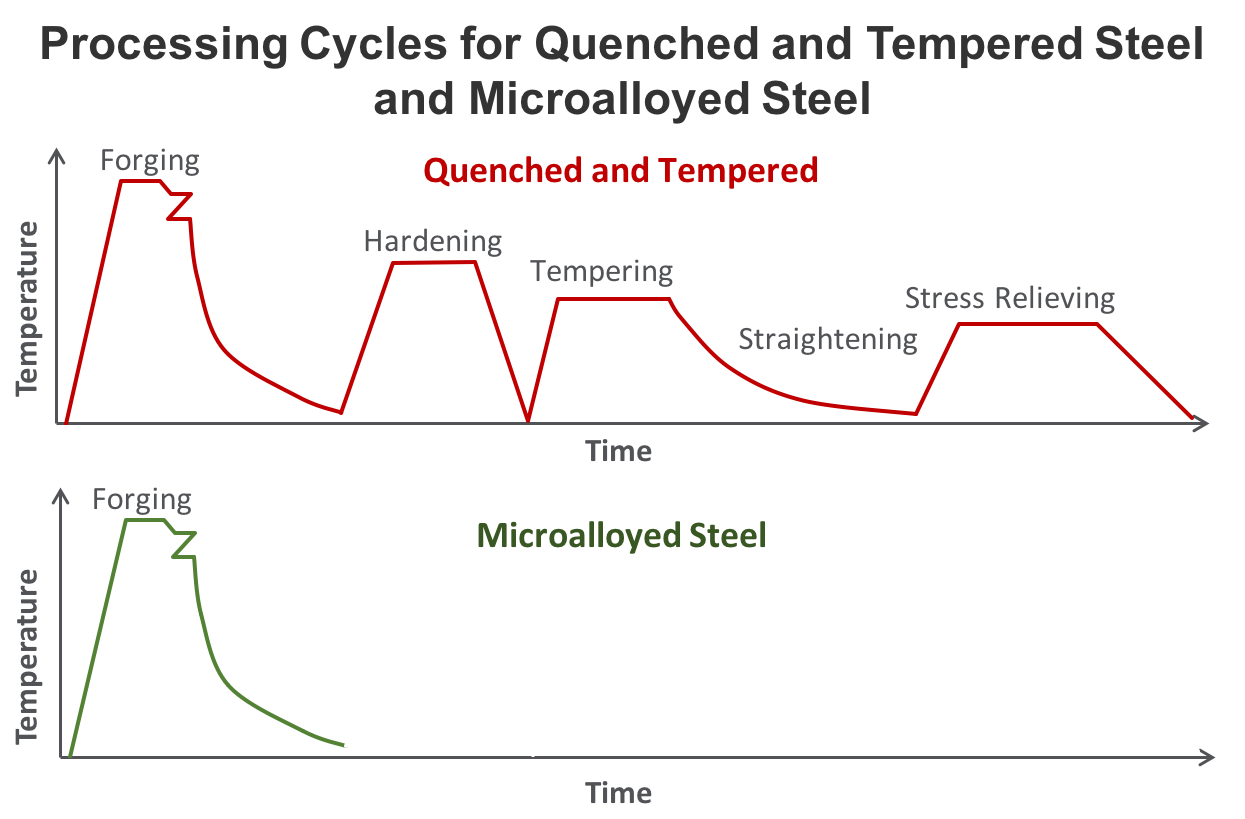
Over 85% of vanadium consumption globally is in the steel industry where it is used primarily in the form of ferro-vanadium. Ferrovanadium is an alloy for high low-alloy strength steels “HSLA” including rebar, pipeline, tool steels, jet engines, axles & crankshafts and reinforcing bars for the construction industry. In low concentrations vanadium can significantly increase the strength and hardness of the steel.
Steel

- Close to 75% of vanadium is produced as ferrovanadium, for high strength low alloy (HSLA) steels used in construction and rebar.
- Vanadium increases resistance to corrosion in tool steels (i.e. axles and crankshafts), for tubes and pipes manufacturing, and in the automotive industry to make components such as hoods, door panels and piston rods.
Master Alloys

- Mixed with aluminum, to strengthen and promote thermal stability in titanium alloy; largely utilized in the aviation sector to produce jet engines, airframes and spacecraft, the high strength to weight ratio provides fuel efficiency.
- Used in nuclear reactors because of the element’s low neutron absorption abilities and resistance to high-temperature stress.
Emerging Applications
Emerging application summary
A highlight emerging application is energy storage using vanadium redox flow batteries (VRFBs). VRFBs are a class of flow batteries known as renewable energy storage systems that offer reliable, long-duration storage without degradation or thermal runaway for large-scale applications including renewable integration, backup storage for critical services and power grids.
Flow Batteries

- Stores energy in liquid vanadium electrolyte (both the anolyte and catholyte) that never degrades. Hardware can be recycled and the vanadium electrolyte (up to 80% of the VRFB) can be reused indefinitely.
- Potential to revolutionize entire power grids and many new applications with sustainable energy storage.
Vanadium Pricing
Vanadium pricing summary
Vanadium is not currently traded on any commodity exchanges such as the LME. Prices are settled in private negotiations between sellers and buyers. Historically, there have been periods of vanadium price volatility due to fluctuations in the supply/demand balance. Prices have ranged from as low as US$4/lb V2O5 to more than US$30/lb V2O5.
Monthly average prices for V2O5 have increased materially since January 2016. This is largely due to low vanadium prices affecting industry shutdowns between 2014 – 2016 that resulted in a significant decrease in global production. Since this time, demand for vanadium has grown steadily, and pricing is relatively stable. Global inventory continues to decrease with most vanadium resource prospects remaining uneconomic or unviable with current pyrometallurgical methods. High technical and capital risks are associated with building a conventional roasting or smelting facility in most global jurisdictions. In addition, the environmental impacts are significant with a carbon footprint of ~ 1.6-2 MT per MT of V2O5 product. This may likely increase carbon penalties and regulatory constraints in the future. The few primary producers in operation (soda ash roasting) and co-product smelters globally must deal with the continuing challenges associated with high environmental costs and limited recovery. Potential solutions for increasing sustainability of vanadium recovery in the future include hydrogen technologies and hydrometallurgical process technologies such as the patented VanadiumCorp Electrochem Process Technology solution wholly owned by VanadiumCorp.
For Current Vanadium Prices (updated weekly) click here